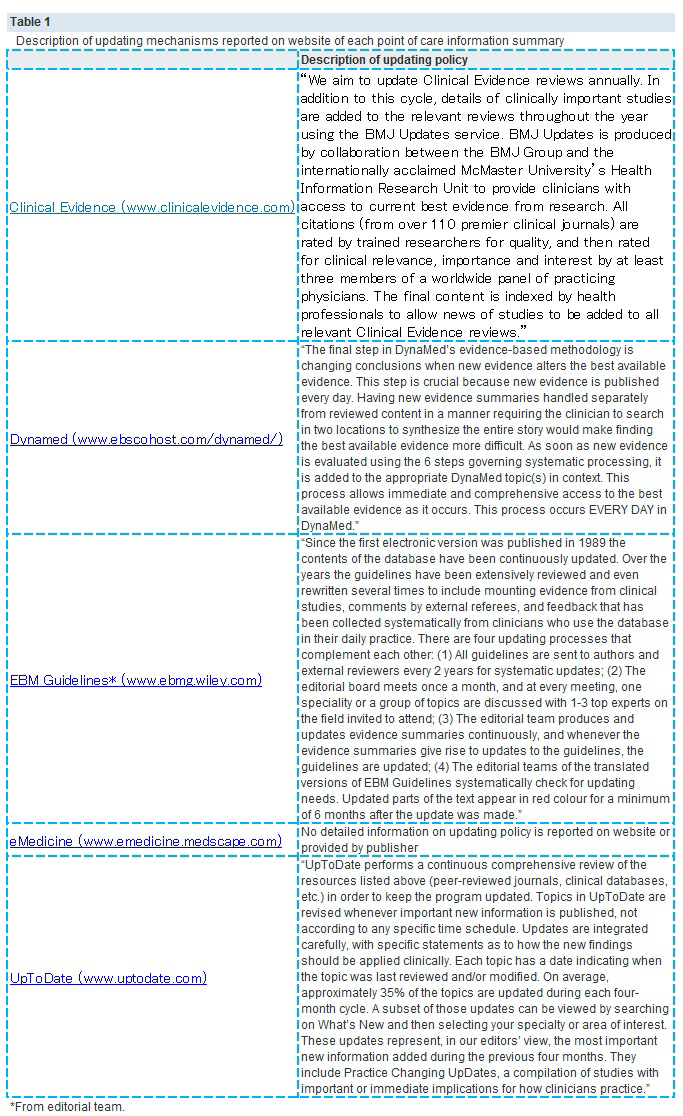
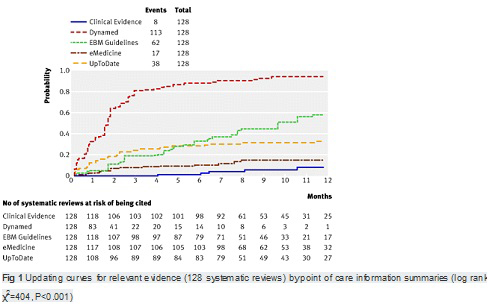
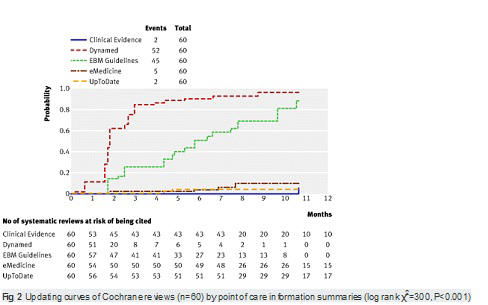
【文献名】
文献タイトル:Speed of updating online evidence based point of care summaries: prospective cohort analysis
雑誌名・書籍名:BMJ 2011
発行年:343:d5856
【要約】
<Objective>
To evaluate the ability of international point of care information summaries to update evidence relevant to medical practice.
<Design>
Prospective cohort bibliometric analysis.
<Setting>
Top five point of care information summaries (Clinical Evidence, EBMGuidelines, eMedicine, Dynamed, UpToDate) ranked for coverage of medical conditions, editorial quality, and evidence based methodology.
<Main outcome measures>
From June 2009 to May 2010 we measured the incidence of research findings relating to potentially eligible newsworthy evidence. As samples, we chose systematic reviews rated as relevant by international research networks (such as, Evidence-Based Medicine, ACP Journal Club, and the Cochrane Collaboration). Every month we assessed whether each sampled review was cited in at least one chapter of the five summaries. The cumulative updating rate was analysed with Kaplan-Meier curves.
<Results>
From April to December 2009, 128 reviews were retrieved; 53% (68) from the literature surveillance journals and 47% (60) from the Cochrane Library. At nine months, Dynamed had cited 87% of the sampled reviews, while the other summaries had cited less than 50%. Overall, 114 systematic reviews (89%) had been cited by at least one point of care summary. The median follow-up time was 33 weeks (range 1-60). Table 2. reports the proportions of citations by summaries over time and the hazard ratio for each summary compared with the top performer. The updating speed of Dynamed clearly led the others. For instance, the hazard ratios for citations in EBM Guidelines and Clinical Evidence versus the top performer were 0.22 (95% confidence interval 0.17 to 0.29) and 0.03 (0.01 to 0.05). This means that the updating speed of Dynamed is 78% and 97% greater than those of EBM Guidelines and Clinical Evidence, respectively. The
median time to citation was 7.7 weeks (range 7-8.2) for Dynamed and 42 weeks (range 34-maximum not reached) for EBM Guidelines.
<Limitations>
Firstly, the total number of citations in the point of care information products should have been taken into account. Secondly, citational analysis counts only bibliographic references without going deeply into the content of the citation. Qualitative analysis of the updating process and how new evidence is incorporated and affects recommendations should also be taken into account in assessing whether one summary is better than others. We did not directly assess how many systematic reviews in our sample called for a change in clinical practice.
Finally, we did not consider the updating of results from studies with other designs (such as randomised clinical trials) as we think that systematic reviews are preferable to support decision making at the point of care..
<Conclusions>
Other studies have assessed other dimensions such as user’s satisfaction, how well different online point of care services answered questions arising in daily clinical work, content development, and evidence based soundness. Updating is only one aspect of the overall quality of a point of care product. But, point of care information summaries include evidence relevant to practice at different speeds. A qualitative analysis of updating mechanisms is needed to determine whether greater speed corresponds to more appropriate incorporation of new information.
【考察とディスカッション】
This literature cannot say which point of care summaries is superior to others. But, it is useful for me to know the difference of the Top 5 point of care summaries.
I’m getting interest in the approaches to content development which found in “Reasons for different updating speeds” section.
“The updating process is based on two phases: identifying important new evidence and assessing whether it offers new information that might change recommendations for clinical practice. In addition, a third phase exists in which the new evidence should be included in the “old” body of knowledge. Updating is not only a matter of literature surveillance but implies a critical evaluation of what a new item of knowledge adds to other works and what that means for clinical practice.”
There is no major differences between products in the first phase, but in the second and third phase, how a new evidence is deemed relevant and then incorporated into the body of the summary probably largely dictates the different updating speeds.
This process is similar in mechanism to our practice, I think.
【開催日】
2012年7月18日